Combined Theoretical and Computational Study of the BelousovÃÆâÃâââ¬Ãâââ¬ÅZhabotinsky Chaotic Reaction and Curtius Rearrangement for Synthesis of Mechlorethamine, Cisplatin, Streptozotocin, Cyclophosphamide, Melphalan, Busulphan and BCNU as AntiÃÆâÃâââ¬Ãâââ¬Å Cancer Drugs
A Heidari
A Heidari*
Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA
- *Corresponding Author:
- A Heidari
Faculty of Chemistry
California South University
14731 Comet St. Irvine
CA 92604, USA
Tel: +1-775-410-4974
E-mail: Scholar.Researcher.Scientist@gmail.com
Received date: June 03, 2016; Accepted date: June 07, 2016; Published date: June 12, 2016
Citation: Heidari A. Combined Theoretical and Computational Study of the Belousov– Zhabotinsky Chaotic Reaction and Curtius Rearrangement for Synthesis of Mechlorethamine, Cisplatin, Streptozotocin, Cyclophosphamide, Melphalan, Busulphan and BCNU as Anti–Cancer Drugs. Insights Med Phys. 2016, 1:2
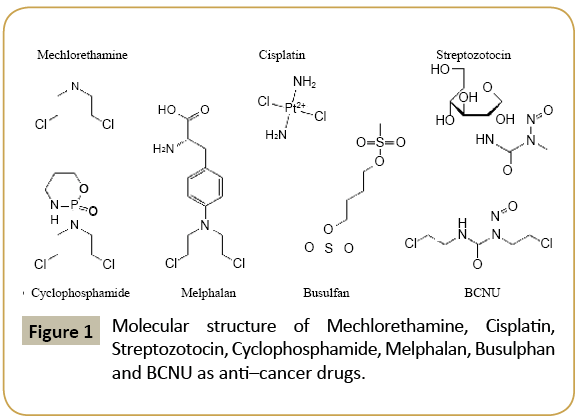
Belousov-Zhabotinsky is a chaotic reaction with a special period for synthesis of some anti-cancer drugs such as Mechlorethamine, Cisplatin, Streptozotocin, Cyclophosphamide, Melphalan, Busulphan and BCNU (Figure 1). Mathematical modeling of this reaction yields three non-linear differential equations which do not have analytical solutions. By numerical simulation (solving the equations numerically) of this reaction, the qualitative behavior of the reaction is specified, which clearly shows the oscillation of reaction [1-17]. On the other hand, Mechlorethamine, Cisplatin, Streptozotocin, Cyclophosphamide, Melphalan, Busulphan and BCNU (Figure 1) can be converted to the other new anti-cancer drugs. The reactions were happen by Curtius rearrangement. Using ab initio and density functional theory (DFT) computational methods and also different basis sets, some structural, thermodynamic and spectroscopic parameters such as electronic energy, polarizability, hardness and electrophilicity values are calculated for the reactants and products of the above mentioned rearrangements. It should be noted that all calculations are carried out by Gaussian 09. Geometry optimization for each molecule are be fulfilled at HF, PM3, MM2, MM3, AM1, MP2, MP3, MP4, CCSD, CCSD(T), LDA, BVWN, BLYP and B3LYP computational methods with 31G, 6-31G*, 6-31+G*, 6-31G(3df, 3pd), 6-311G, 6-311G* and 6-311+G* basis sets, respectively. The change of the hardness, polarizability and electrophilicity index during these reactions are calculated. It is shown that the maximum hardness, minimum polarizability and minimum electrophilicity principle are valid in all reactions. These results show the validation of this new principle in Curtius rearrangement [18-29].
References
- Vijayajayanthi M, Kanna T, Lakshmanan M, Murali K (2016) Explicit construction of single input–single output logic gates from three soliton solution of manakov system. Communications in nonlinear science and numerical simulation 36: 391-401.
- Arenas LF, Ponce de León C, Walsh FC (2016) Electrochemical redox processes involving soluble cerium species. Electrochimica Acta 205: 226-247.
- Minoru Y, Taishi M, Mutsuo T, Shigeru K (2016) Determination of thermodynamic parameters for enolization reaction of malonic and metylmalonic acids by using quartz crystal microbalance. Anal Chem Res 8: 9-15.
- Per Sebastian S, Dane T, Jie S, Alex A (2016) Erosion of synchronization: Coupling heterogeneity and network structure. 323: 40-48.
- Bomfim T, Castro A, Varandas P (2016) Differentiability of thermo-dynamical quantities in non-uniformly expanding dynamics. Adv Math 292: 478-528.
- György S, István B (2016) Evolutionary potential games on lattices. Phys Rep 624: 1-60.
- Tse G (2016) Mechanisms of cardiac arrhythmias. J Arrhythmia 32: 75-81.
- Juan C, Lin H, Gang H, Zhang Y, Yuanyuan H, et al. (2016) An application of chemical oscillation: distinguishing two isomers between cyclohexane-1,3-dione and 1,4-cyclohexanedione. Electrochimica Acta 195: 223-229.
- McFarlane C, Paul G, Thomas B, Liming Y (2016) Efficient lipid peroxidation catalyzed by amyloid-beta-copper complex: observation of chemical oscillation and chaos. Biophys J 110: 552.
- Tsiairis CD, Aulehla A (2016) Self-organization of embryonic genetic oscillators into spatiotemporal wave patterns. Cell 164: 656-667.
- Yuuka F, Kunihiko O, Taisuke B, Kouichi A (2016) Controlled polymerization of acrylonitrile proceeded along with the Belousov–Zhabotinsky oscillator by changing its stirring conditions. Chem Phys Lett 645: 210-214.
- Sabine HLK (2016) Collective dynamics of dipolar and multipolar colloids: From passive to active systems. Curr Opin Colloid In 21: 76-85.
- Philsu K, Junghan K, WonKyu J, Sunyoung B (2016) An error embedded method based on generalized Chebyshev polynomials. J Comput Phys 306: 55-72.
- Tsukuru M, Aya MA, Ryo Y (2016) Self-oscillating polymer materials. In: Biomaterials Nano architectonics. Mitsuhiro E, (ed.) William Andrew Publishing 219-236.
- Martin D, Maxime N, Véronique S, Rénal B (2016) Integrative chemistry: Positioning chemical reactors within the geometric space as a tool for the design of advanced functional materials. Comptes Rendus 19: 216-230.
- Brown P, Eral HB (2016) Smart and stimuli-responsive colloids. In: Nanocolloids. Domínguez MS, Rodríguez AC (eds.) Elsevier Amsterdam pp: 389-426.
- Nakane K, Mahara H, Kida K (2016) An image analyzing method for the vaguely grain boundary detection by a reaction diffusion system. Proc Mate Sci 12: 72-76.
- Xianyu S, Rachita R, Jeffrey RD, MacKerell AD, Fengtian X, et al. (2014) Boc-protected 1-(3-oxocycloalkyl) ureas via a one-step curtius rearrangement: mechanism and scope. Tetrahedron Lett 55: 842-844.
- Kaori H, Tominari C, Kyoko C, Aimi O, Rikako Y, et al. (2012) A novel total synthesis of isocryptolepine based on a microwave-assisted tandem curtius rearrangement and aza-electrocyclic reaction. Tetrahedron 68: 4274-4279.
- Maxim VZ, Roald PT (2010) Why Lewis acids accelerate the thermal Curtis rearrangement of benzoyl azide into phenyl isocyanate. J Mol Struc Theochem 962: 15-22.
- Matthew LL, Emily AP (2010) Facile preparation of protected benzylic and heteroarylmethyl amines via room temperature curtius rearrangement. Tetrahedron Lett 51: 2888-2891.
- Santoshi S, Mitsuru O (2009) Solid-phase synthesis of aryl, heteroaryl, and stearically hindered alkyl amines using the Curtius rearrangement. Tetrahedron 65: 638-643.
- Daisuke S, Shinya S, Hideyo T, Shiro I (2008) Novel synthesis of oligosaccharides linked with carbamate and urea bonds utilizing modified curtius rearrangement, Tetrahedron, 64: 8780-8788.
- Christopher AL, Millichip I, Beth P, James R, Mark F (2007) A convenient synthesis of sulfonylureas from carboxylic acids and sulfonamides via an in situ curtius rearrangement. Tetrahedron Lett 48: 8878-8882.
- Jun-ichi M, Masahiko O, Kosuke T, Hiroyuki T, Hiroyuki I (2007) A practical synthesis of enantiopure N-carbobenzyloxy-N'-phthaloyl-cis-1,2-cyclohexanediamine by asymmetric reductive amination and the curtius rearrangement. Tetrahedron Asymmetr 18: 1906-1910.
- Kenzo Y, Shin K, Yutaka S, Motomu K, Masakatsu S (2007) A concise synthesis of Tamiflu: third generation route via the Diels-Alder reaction and the curtius rearrangement. Tetrahedron Lett 48: 1403-1406.
- Daisuke S, Shinya S, Hideyo T, Shiro I (2006) A new and facile synthesis of carbamate and urea linked glycoconjugate using modified curtius rearrangement. Tetrahedron Lett 47: 7219-7223.
- Gómez-Sánchez E, Marco-Contelles J (2005) Synthesis and transformations of alkyl N-(1-cyclohex-3-enyl) carbamates prepared from cyclohex-3-ene carboxylic acid via Curtis rearrangement. Tetrahedron 61: 1207-1219.
- Patrick HD, Chunping X (2004) Curtius rearrangement and Wolff homologation of functionalized peroxides. Tetrahedron Lett 45: 7455-7457.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences